Background
CYAD-01 is a T-cell product engineered to express a chimeric antigen receptor (CAR) based on the NKG2D receptor (NKG2D CAR) which binds 8 ligands (MICA/B, ULBP1-6) over-expressed by a large variety of malignancies, including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).
The phase I THINK study (NCT03018405) evaluated the safety and clinical activity of multiple injections of CYAD-01 infused every 2 weeks, without preconditioning chemotherapy, in 13 relapsed/refractory (r/r) AML and MDS patients. While an encouraging objective response rate according to ELN2017 (AML) or revised IPSS (MDS) and reduction in bone marrow blasts were seen with good safety profile, the responses were short-lived (≤ 3 months - see ASH 2019, poster 3826). To enhance CAR T-cell persistence, we evaluated a weekly dose schedule without preconditioning (THINK study) or the addition of cyclophosphamide and fludarabine (CyFlu) as a preconditioning regimen prior to CAR T-cell infusion (phase I DEPLETHINK study, NCT03466320).
Aim
To further increase persistence and potency of the T-cell product, optimization of the previously used mAb manufacturing process was performed by shortening the duration of production along with modification of PI3K inhibitor. This optimized manufacturing process (termed "OptimAb") aimed to generate CYAD-01 cells with a higher frequency of early memory T-cells with high cytokine secretion upon activation, as compared to the original "mAb" process.
Results
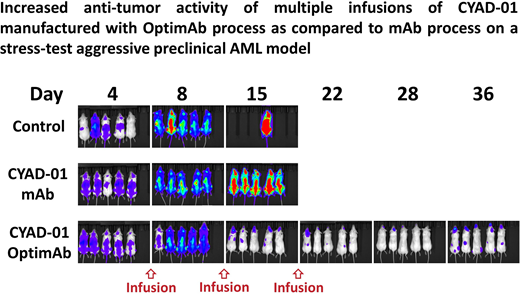
As compared to the previous mAb manufacturing process, the OptimAb manufacturing process generates a product that secretes higher levels of IFN-γ upon co-culture with tumor cells and contains a higher frequency of CD62L+ T-cells in vitro, characteristic of an early memory phenotype. In an in vivo aggressive AML (THP-1) model, CYAD-01 OptimAb displayed a strong improvement in long-term anti-tumor activity as compared to the CYAD-01 mAb at the same dose chosen to have a minimal anti-tumor activity (stress-test dose, see figure).
Based on these results, both THINK and DEPLETHINK clinical studies were amended to evaluate the OptimAb process. As of August 2020, 5 patients have been treated with multiple infusions of the OptimAb CYAD-01 as standalone treatment at the dose of 3x108 cells/infusion in the small expansion segment of the THINK study. 7 patients were treated with a single infusion of OptimAb CYAD-01 administered after a CyFlu preconditioning in the dose-escalation segment at the doses of 3x108 cells/infusion or 1x109 cells/infusion in the DEPLETHINK study. To date, the results demonstrate the safety and tolerability for CYAD-01 OptimAb with or without a prior lymphodepletion in patients with r/r AML and MDS.
Preliminary data of the clinical and pharmacokinetics evaluation of CYAD-01 manufactured with the improved OptimAb process, as compared with the mAb process at the same dose, in two Phase I studies will be provided at the time of presentation.
Conclusion/summary
The autologous CYAD-01, a first generation NKG2D CAR T-cell product is currently investigated in r/r AML/MDS patients, a difficult to target disease due in part to the absence of truly AML-specific surface antigens, its rapid clinical progression and the absence of disease control by the CyFLu preconditioning. CYAD-01 manufactured using an optimized process, OptimAb, aims to improve CAR T-cell persistence and clinical responses. The data analysis of the same CAR-T product with different manufacturing processes, with or without preconditioning chemotherapy, will provide the medical community with clinical and scientific insights to guide the future of this therapeutic modality.
Sallman:Agios, Bristol Myers Squibb, Celyad Oncology, Incyte, Intellia Therapeutics, Kite Pharma, Novartis, Syndax: Consultancy; Celgene, Jazz Pharma: Research Funding. Al-Homsi:Celyad: Membership on an entity's Board of Directors or advisory committees. Pollyea:Janssen: Consultancy; 47: Consultancy, Research Funding; Amgen: Consultancy; Genentech: Consultancy; Novartis: Consultancy; Karyopharm: Consultancy; Syndax: Consultancy; Syros: Consultancy; Abbvie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Takeda: Consultancy; Pfizer: Consultancy; Celgene/BMS: Consultancy; Agios: Consultancy; Glycomimetics: Other. Wang:Abbvie: Consultancy; Pfizer: Speakers Bureau; Genentech: Consultancy; Stemline: Speakers Bureau; PTC Therapeutics: Consultancy; Macrogenics: Consultancy; Astellas: Consultancy; Bristol Meyers Squibb (Celgene): Consultancy; Jazz Pharmaceuticals: Consultancy. Demoulin:Celyad Oncology: Current Employment. Sotiropoulou:Celyad Oncology: Current Employment. Alcantar-Orozco:Celyad Oncology: Current Employment. Breman:Celyad Oncology: Current Employment. Dheur:Celyad Oncology: Current Employment. Braun:Celyad Oncology: Current Employment. Lonez:Celyad Oncology: Current Employment. Gilham:Celyad Oncology: Current Employment. Flament:Celyad Oncology: Current Employment. Lehmann:Celyad Oncology: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal